
Little sensor. Big deal.
Minimizing device frustrations and achieving a YEAR of reliable, accurate glucose monitoring takes science and innovation. See what makes Eversense 365 different.

One CGM. One year of minimal disruptions1-3 with just:
- One sensor per year
- One transmitter per year
- One fingerstick calibration per week*
With just:
- One prescription per year
- One approval submission per year
How the Eversense 365 system works
This tiny sensor turns glucose molecules into light signals—all day, every day, for a YEAR. Here’s how.
The Eversense 365 System
Eversense 365 is a long-term solution to the everyday frustrations of traditional, short-term CGMs.
One year sensor is placed under the skin of upper arm.


Gentle silicone-based adhesive secures transmitter over the skin.


Removable† smart transmitter powers the sensor and receives glucose data.
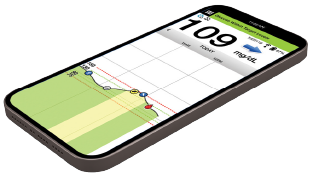
Smart transmitter sends glucose data to mobile app.
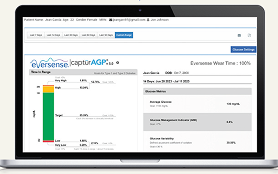
Healthcare providers can view data reports on DMS Pro.
Images are for illustration and may not show actual proportions of system components
Eversense 365 helps solve the real-life challenges of
traditional short-term CGMs
(at 60 mg/dL)
Ready to Get Started?
Ready to start prescribing? Learn how simple it can be. Learn how to become an Eversense Inserter.

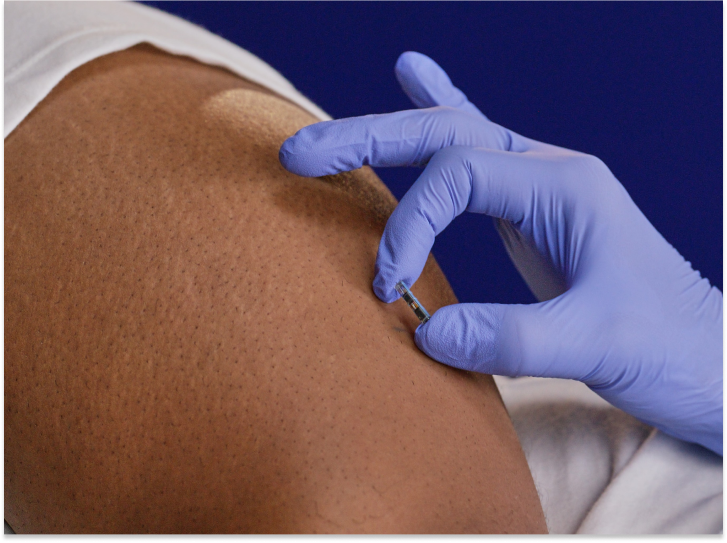
The sensor placement procedure
See how simple sensor placement is for patients. Our video shows what they can expect during the brief, in-office appointment.
* After day 13
† There is no glucose data generated when the transmitter is removed
1. Senseonics. (2024) Eversense 365 Continuous Glucose Monitoring System User Guide. LBL-7702-01-001
2. Dexcom (2025) G7 User Manual AW00078-10 Rev 004 MT-00078-10
3. Abbott. (2024) Freestyle Libre 3 Continuous Glucose Monitoring System User Guide ART49385-001 Rev. A 04/24
4. Christiansen MP et al. (2018) A Prospective Multicenter Evaluation of the Accuracy of a Novel Implanted Continuous Glucose Sensor: PRECISE II. DIABETES TECHNOLOGY & THERAPEUTICS, 20(3):197-206
5. Deiss, D. et al. (2020). Real-world safety of an implantable continuous glucose sensor over multiple cycles of use: A post-market registry study. Diabetes Technology and Therapeutics, 22(1), 48–52. https://doi.org/10.1089/dia.2019.0159
Disclaimer
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001709 Rev 1