Mark
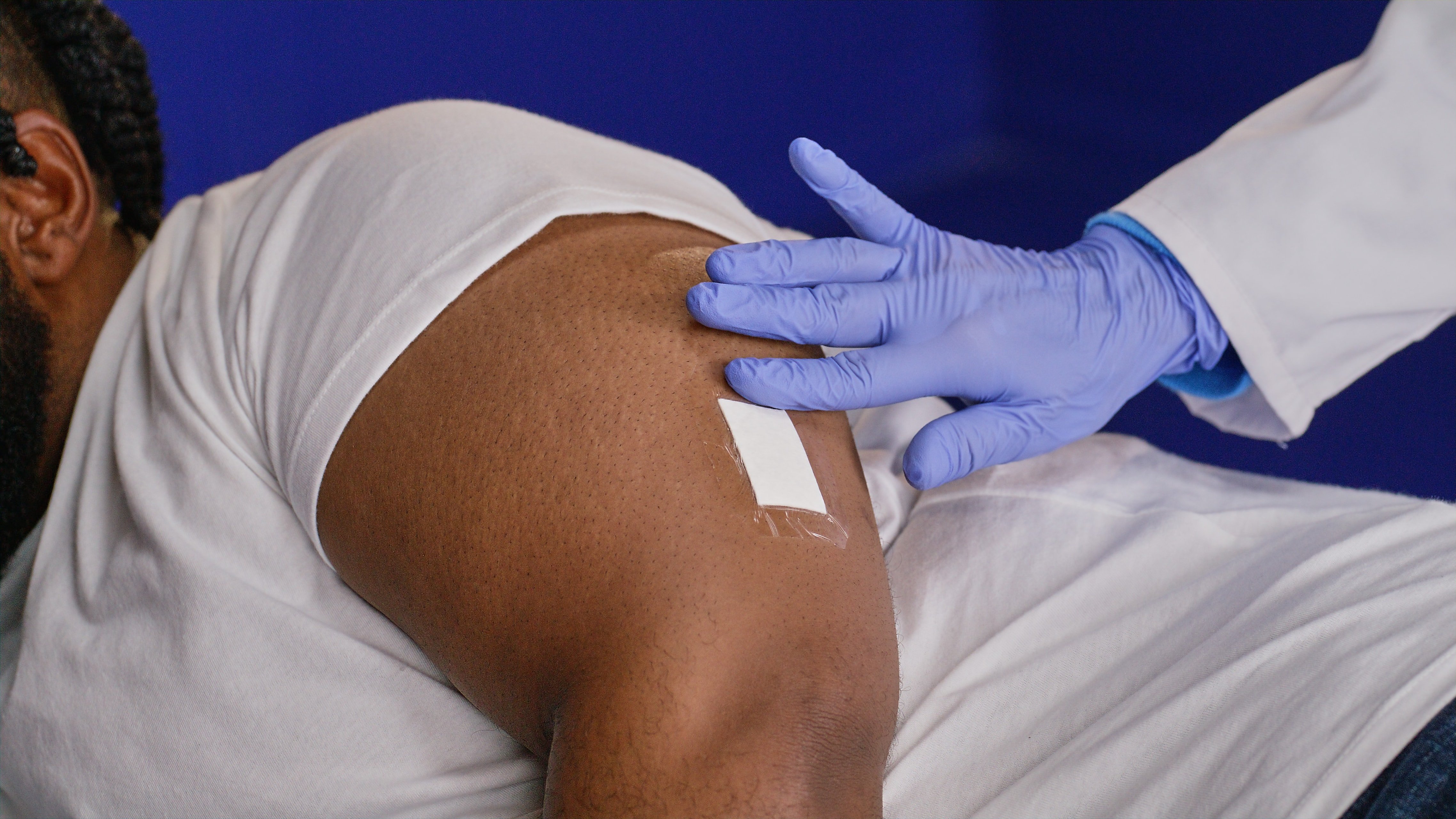
Site is chosen and marked in the upper arm

One simple procedure is all it takes to get Eversense 365
You prescribe Eversense 365 and a trained HCP in the Eversense Inserter network takes care of the sensor placement. Patients can get the sensor placed over a lunch break if they want to.
See how simple sensor placement is for patients. We review every detail in our video.
Placement takes just a few minutes. No sutures are necessary. Patients can get back to their regular routine immediately.*
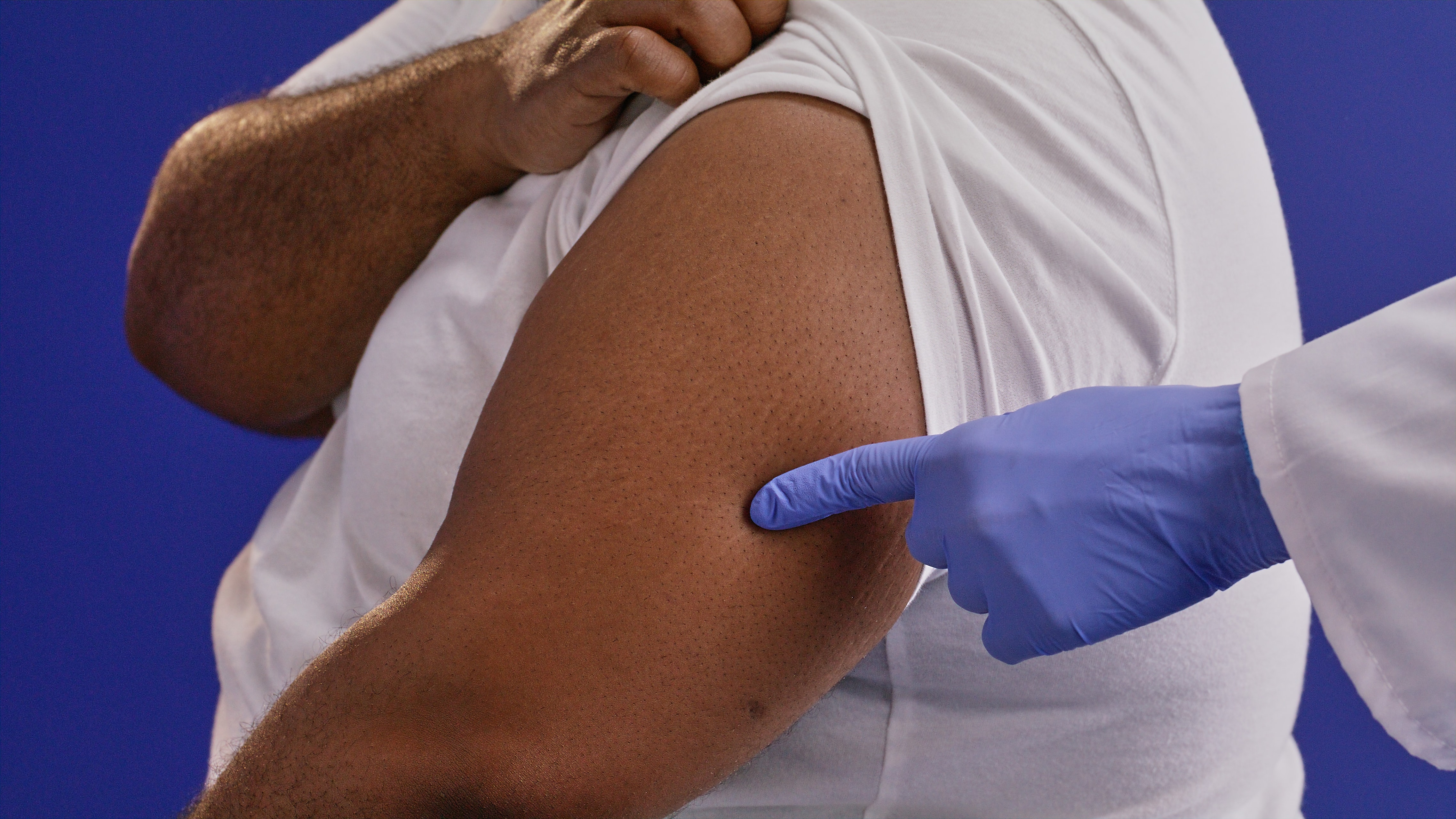
Site is chosen and marked in the upper arm
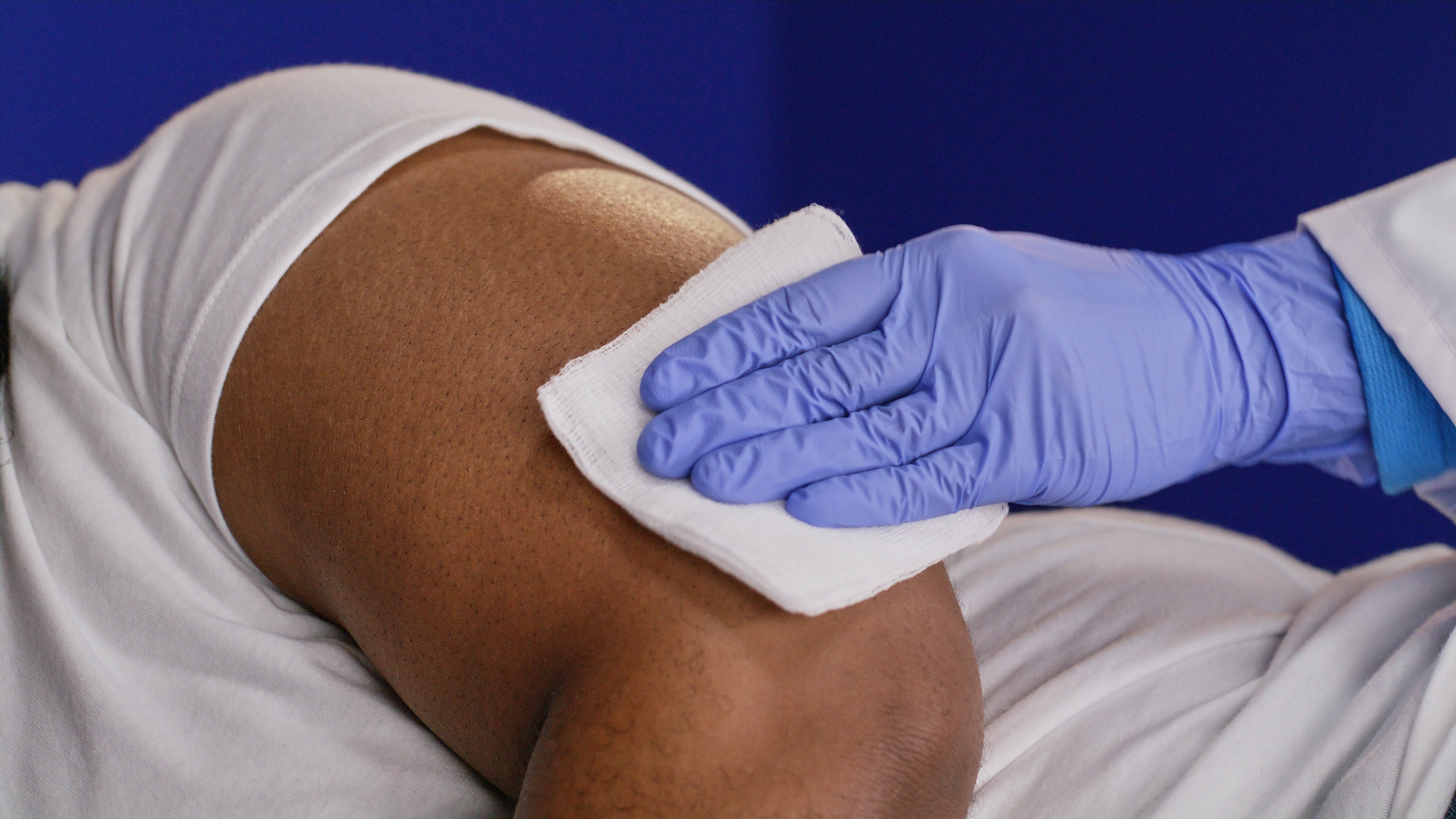
Sterile field is created, area is cleansed, and a local anesthetic is injected
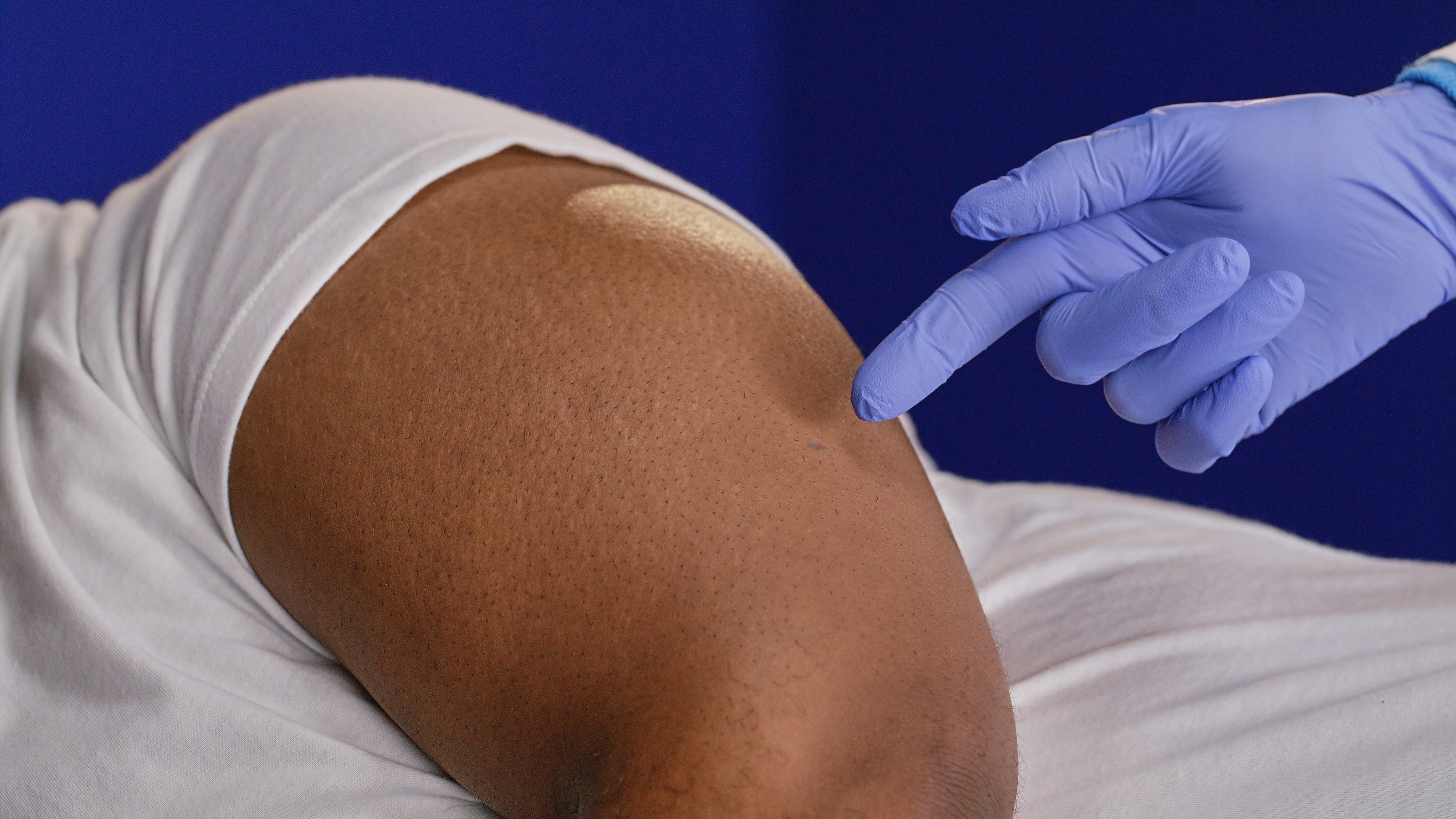
A ~5mm incision is made, just large enough for the sensor’s tiny width
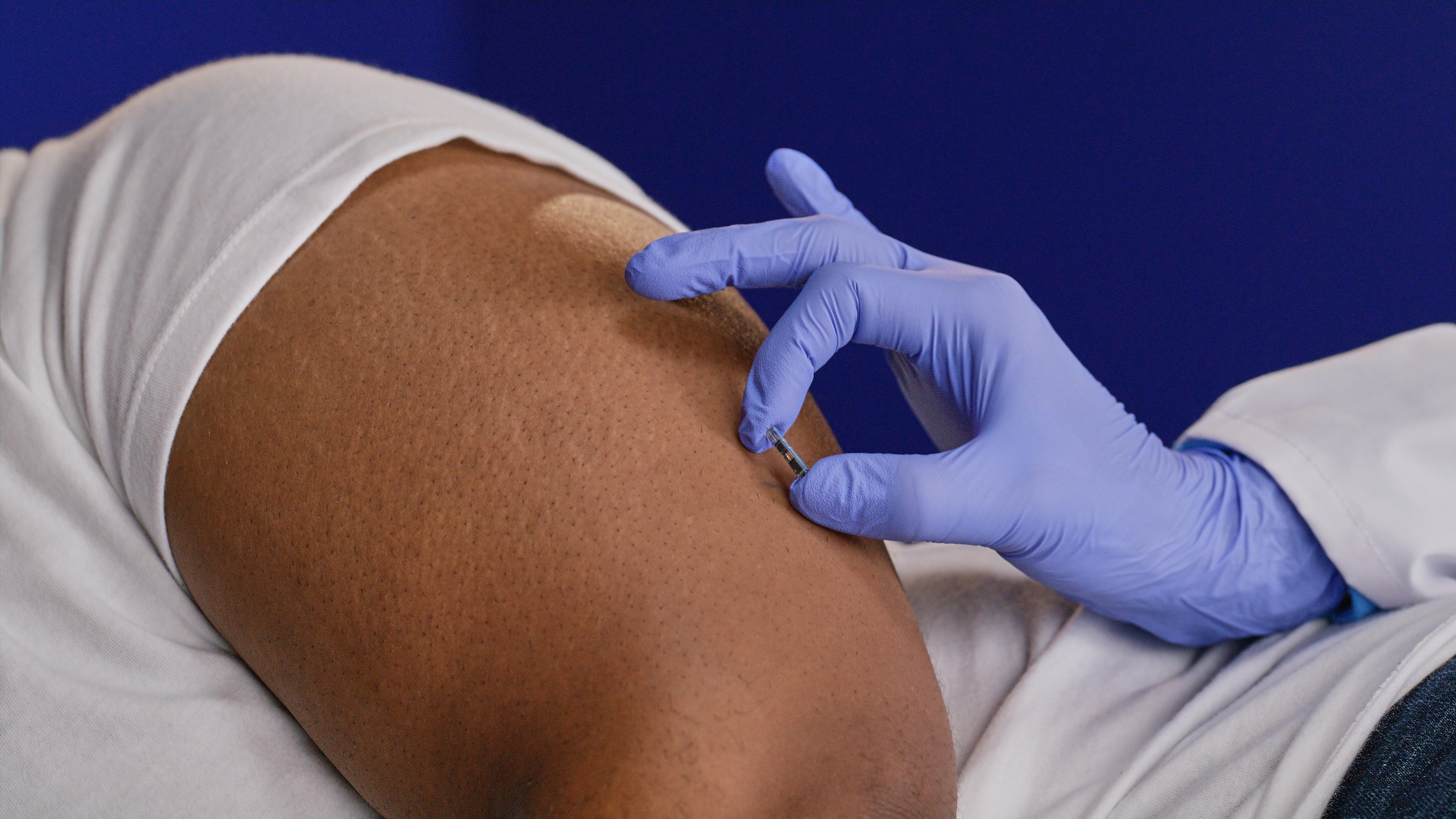
A small subcutaneous pocket is made 3-5 mm below the skin's surface
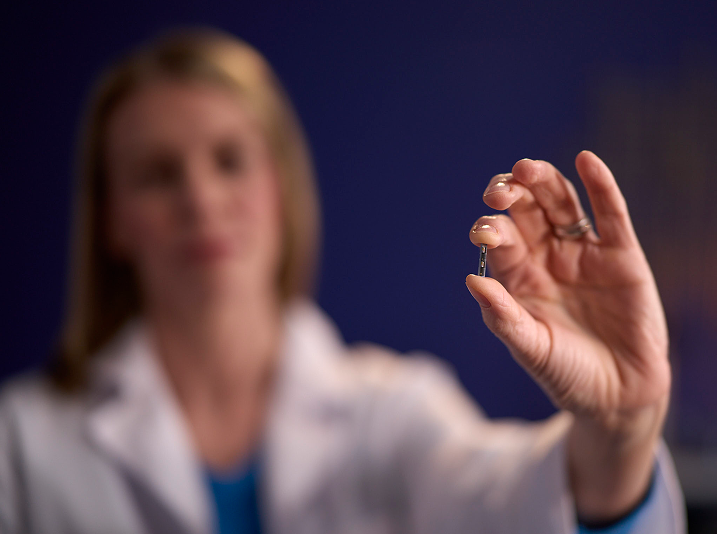
The sensor is placed with a custom insertion tool designed for precise placement

The incision is closed with Steri-StripsTM and TegadermTM
After the sensor placement
You continue to manage your patients, just as you would if you prescribed a traditional CGM. After a year, patients return to an Eversense Inserter to get the old sensor removed and a new one placed in the other arm, same as before.

The Eversense Inserter network
handles sensor placement
Skilled medical professionals who will manage:
Ready to Become a
Provider?
Learn more about what steps you can take to prescribe Eversense 365 or how to become an Eversense Inserter.
† In the majority of cases
* Avoid strenuous activities that may pull at the incision or cause a lot of sweating, around the insertion area while the incision heals. Do not swim or soak in a tub for five days.
1. Bailey TS, et.al., Evaluation of Accuracy and Safety of the 365-Day Implantable Eversense Continuous Glucose Monitoring System: The ENHANCE Study. Diabetes Technology & Therapeutics, 2025 Jan 27. doi: 10.1089 / dia.2024.0592.
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.
MKT-001711 Rev 1