Longest Lasting CGM1-3
Eliminates frequent CGM changes with only one day 1 or warm up period per year.

ONE YEAR. ONE CGM.
Introducing Eversense 365. Help your patients manage diabetes, not device frustrations.
See how Eversense 365 outlasts and outperforms traditional CGMs.


Eliminates frequent CGM changes with only one day 1 or warm up period per year.

Most reliable detection of hypoglycemic events, with discreet on-body vibration alerts.

Removable* smart transmitter can be taken off when desired and put back on without wasting a CGM.
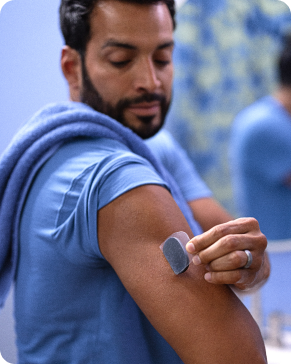
Almost no skin reactions5 with silicone-based adhesive.
Patient referrals can be made to Ascensia Diabetes Care by:
• Parachute Health
or
• Fax Order for Therapeutic Implantable CGM (medical necessity documentation), Chart Notes and Insurance Info
Our skilled medical professionals will manage:
• Insertion and removal procedures
• Billing
• Scheduling

For patients with Commercial Insurance
Eversense 365 is covered by most commercial health plans.6
Eligible patients may pay as little as $199 for a full year with the Eversense PASS Program†.

Deepen your knowledge to enhance patient discussions
1. Senseonics. (2023) Eversense E3 Continuous Glucose Monitoring System User Guide.: LBL-6002-01-001
2. Senseonics. (2024) Eversense 365 Continuous Glucose Monitoring System User Guide. LBL-7702-01-001
3. Dexcom (2024) G7 User Manual AW00078-10 Rev 003 MT-00078-10
4. Abbott. (2024) Freestyle Libre 3 Continuous Glucose Monitoring System User Guide ART49385-001 Rev. A 04/24
5. Deiss D., et al. Real-World Safety of an Implantable Continuous Glucose Sensor Over Multiple Cycles of Use: A Post-Market Registry Study. Diabetes Technol & Ther, 2020, 22(1);48-52. DOI: 10.1089/dia.2019.0159
6. Data on File. Senseonics. Policy Reporter (8/23/2024)
7. Not a guarantee of coverage and payment. A doctor’s prescription is required for Medicare coverage and patients must meet Medicare eligibility coverage criteria. Coverage and payment may be subject to coinsurance, deductible and patient eligibility requirements. Visit http://www.medicare.gov to learn more about Medicare plans and coverage.
* There is no glucose data generated when the transmitter is removed
† Offer does not apply to the insertion and removal procedures. The Eversense PASS Program has limitations on eligibility. See website (bit.ly/EversensePass) for more details. Ascensia Diabetes Care reserves the right to change or terminate this program at any time without notice. There is a maximum payment of $2,500 per transaction for the program.. There may be instances in which patient out-of-pocket costs exceed what is detailed in the offer. Eversense E3 is not eligible for the PASS Program.
Disclaimer
The Eversense® E3 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to 180 days in persons with diabetes age 18 and older. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time a day after day 21, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense E3 CGM System is a prescription device; patients should talk to their health care provider to learn more.
The Eversense® 365 Continuous Glucose Monitoring (CGM) System is indicated for continually measuring glucose levels for up to one year in people (18 years and older) with diabetes. The system is indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are required for calibration one time a week after day 13, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense 365 CGM System is a prescription device; patients should talk to their health care provider to learn more.
For important safety information, see bit.ly/eversensesafety
Eversense, Eversense E3 Continuous Glucose Monitoring, Eversense 365 Continuous Glucose Monitoring, and the Eversense logo are trademarks of Senseonics, Incorporated. Ascensia, the Ascensia Diabetes Care logo are trademarks and/or registered trademarks of Ascensia Diabetes Care Holdings AG. All other trademarks are properties of their respective owners and are used solely for informative purposes. No relationship or endorsement should be inferred or implied.
Apple Watch® is a product of Apple, Inc., and may be separately purchased from an authorized Apple retailer. Apple Watch is not included with the Eversense CGM System. Android is a trademark of Google LLC.TCOYD
PP-SENS-E3-GBL-0066